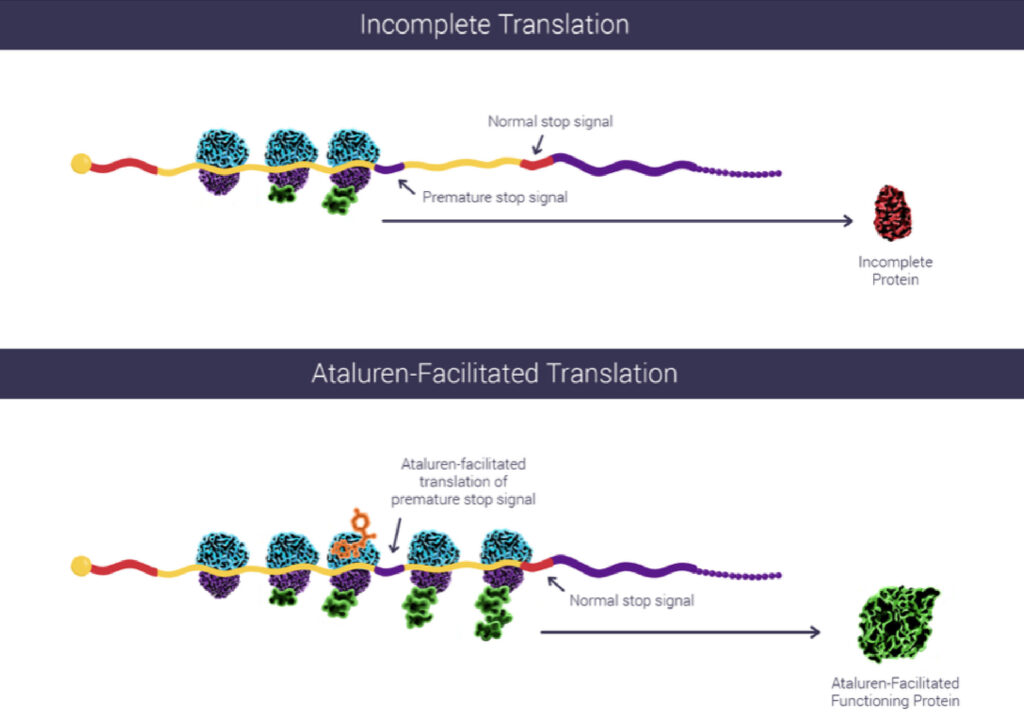
Voorwaardelijke toelating tot basispakket voor Translarna bij Duchenne spierdystrofie - Duchenne Parent Project
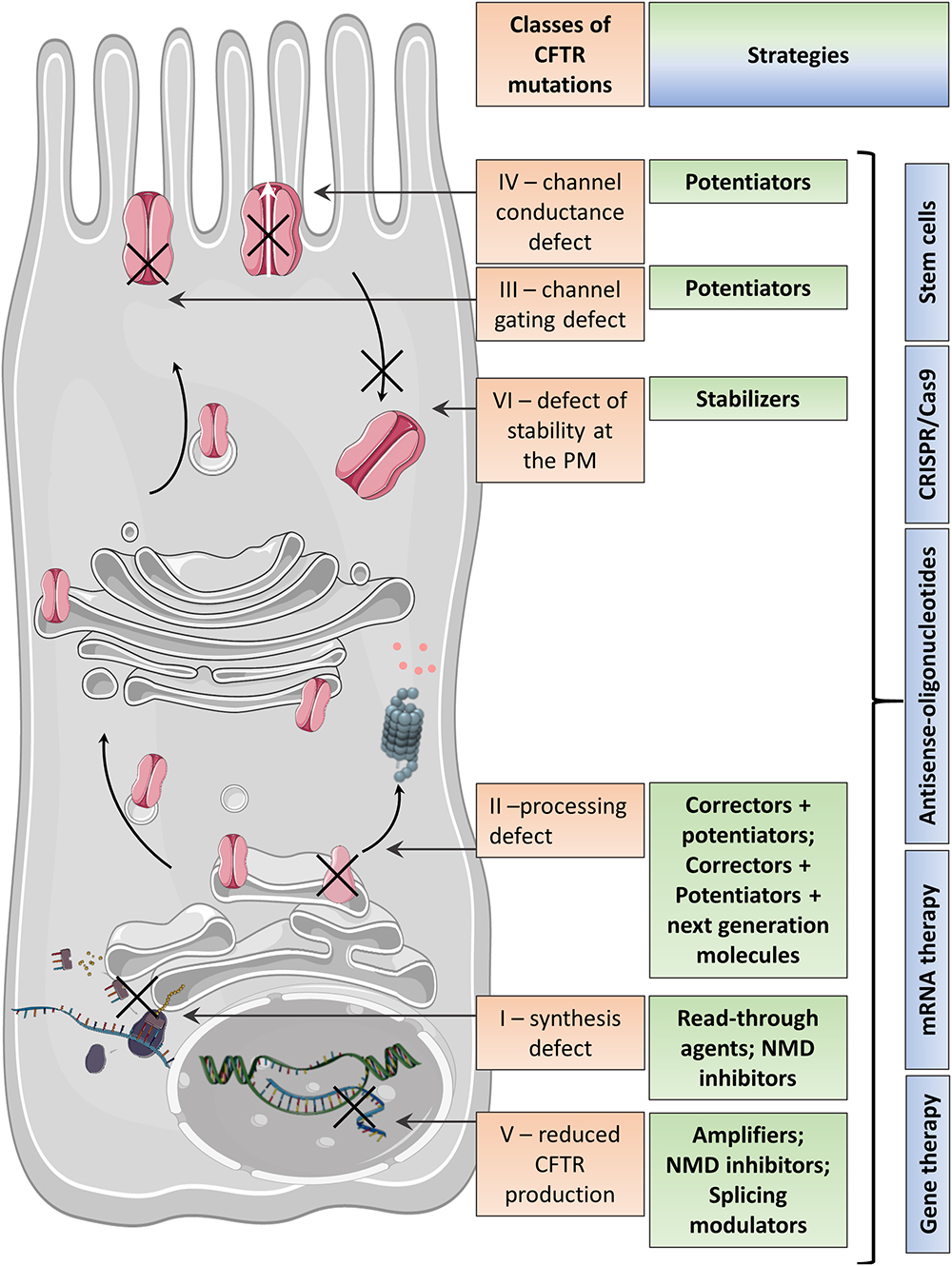
Frontiers | Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine | Pharmacology

Efficacy and safety of ataluren in patients with nonsense-mutation cystic fibrosis not receiving chronic inhaled aminoglycosides: The international, randomized, double-blind, placebo-controlled Ataluren Confirmatory Trial in Cystic Fibrosis (ACT CF ...

Voorwaardelijke toelating tot basispakket voor Translarna bij Duchenne spierdystrofie - Duchenne Parent Project

Tobramycin is a potent inhibitor of ataluren-mediated readthrough. The... | Download Scientific Diagram

Tobramycin is a potent inhibitor of ataluren-mediated readthrough. The... | Download Scientific Diagram















